12. Protein Ligand Interactions:
Protein-Ligand Complex
A complex is created as a result of certain interactions between proteins and ligands.
The function of the protein is inhibited if the ligand is an inhibitor that hits the active site.
Specific Interactions
Non-covalent interactions in particular include:
Electric charge interactions extended range: from 3 to >10 Å atomic
distances, from strong to weak
Donor-acceptor atom distances for hydrogen bonds are between 2.8 Å and
3.5 Å, ranging from strong to weak.
Van der Waals interactions (vdW): weak short range: atomic distances 3–4
Å
Molecular Recognition Model
Partners are drawn to one another by electrostatic interaction (controversial: molecular connections are frequently diffusion-controlled)
The orientation and specificity of the connection are established via hydrogen bonds, which often also increase the strength of the binding.
A tight interface is created via vdW interactions, and the interaction's strength is determined by the resulting "hydrophobic effect."
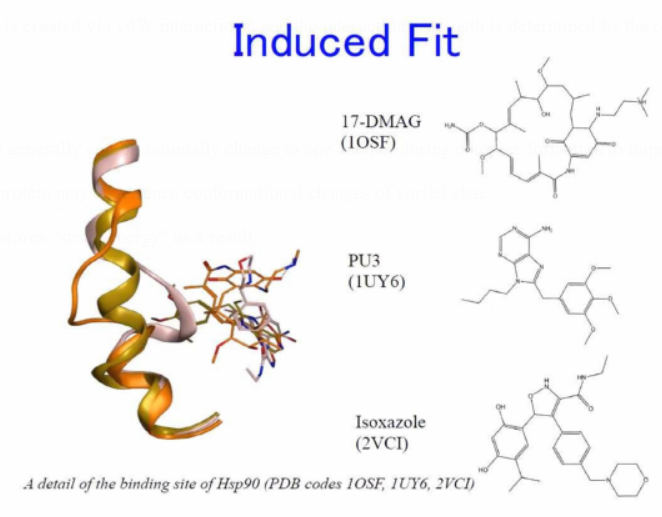
Induced Fit
Binding partners generally conformationally
change to one another during complex formation to improve the interface
("induced fit").
As a result, the protein may experience conformational changes of varied
size.
The ligand also stores "strain energy" as a result.
P+L <==> PL
A thermodynamic equilibrium is reached when a protein-ligand complex forms.
ΔG=ΔH - TΔS
The Gibbs free energy must drop for the equilibrium to move to the complex's side.
ΔG(PL) < ΔG(P) + ΔG(L)
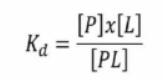
The unit "M" (molar) represents the dissociation constant.
'L' is sometimes referred to as the inhibitory constant 'Ki' if it is an inhibitor.
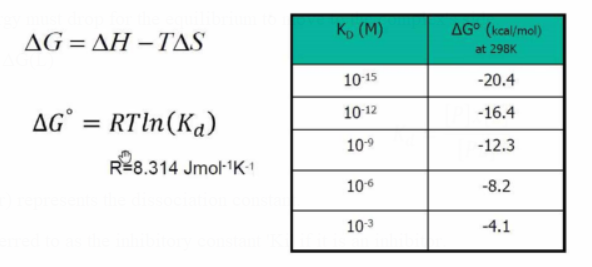
The Gibbs free energy changes by approximately -1.4 kcal/mol for every 10-fold increase in the stability of the PL complex and 10-fold greater P-L interactions, respectively.
To determine the consequences of certain P-L interactions, utilize this value.
Binding Process Components
Any P-L complex formation is often accompanied by the following effects:
-
Ligand must be destroyed, incurring an enthalpy penalty.
-
Enthalpy penalty: Protein must be desolvated.
-
Entropy penalty: Ligand is immobilized upon binding.
-
Ligand strain energy: enthalpic penalty upon binding.
-
Entropy penalty: Protein side chains get immobilized.
-
When protein-ligand interfaces occur, water molecules are released into the solvent due to an enthalpy reduction: Gain in entropy ("hydrophobic effect")
ΔG=ΔH - TΔS
Complexity development depends on:
Positive protein-ligand interactions provide enthalpic contributions.
A hydrophobic reaction: Entropic and/or enthalpic forces may be used to induce ligand binding and complex formation.
Hydrophobic Effect
Proteins' active/binding sites are frequently at
least partially hydrophobic because they must protect ligands from
solvent.
Unused binding sites must be hydrated in solution.
Hydrogen bonding let water molecules create ordered layers at
hydrophobic surfaces.
Water molecules with order have less entropy.
If ligands take the place of ordered fluids, the
ordered waters are discharged into the solvent environment.
The emission results in a significant increase in entropy.
Then, through a variety of interactions, a tight hydrophobic protein-ligand
interface is created.
Hydrophobic vdW interactions often provide less energy to the system
than entropy gain.
A protein-ligand interaction with high surface complementarity is often
a sign of a potent hydrophobic impact.
.png)
Interaction Energies
The energy of electrostatic interactions depend
on distance and
change in response to shielding.
Van der Waals interactions are weak, but if they occur over broad
contacts, they might become significant: around 0.1 to 0.5 kcal/mole
(hydrophobic atoms)
Geometrical restrictions and a wide range of free energy contributions
apply to hydrogen bonds:
around 0–4 kcal/mole
Hydrogen Bonds
For the specificity of protein-ligand
interactions, molecular recognition is frequently essential.
Why do they drastically differ in their contributions to the binding
free energy?
The environment in which they originate and the chemical characteristics
of the donors and acceptors have an impact on the strength of hydrogen
bonding interactions.
Hydrogen Bond Components
Any hydrogen bond creation is often accompanied by the following outcomes:
-
Enthalpy penalty: Polar donor must be desolvated.
-
Enthalpy penalty: Polar acceptor must be desolvated.
-
Entropy penalty: Immobilization of ligand donor/acceptor groups and protein side chains.
-
Enthalpy decrease during H-bond formation.
-
The contact is weakened if the H-bond is partially solvent exposed due to water molecule shielding.
H-Bond
H-bonds can have a substantial or neutral effect
on the free energy of binding, depending on these variables and the
surrounding environment.
Even energetically inert H-bonds add to the interaction's specificity!
This is true because unsaturated H-bond donor or acceptor functions at
protein-ligand interfaces must be solvated and are not allowed.
Salt-Bridges
H-bonds between charge donors and acceptors called salt bridges.
A salt bridge can be
defined as an interaction between two groups of opposite charge in which
at least one pair of heavy atoms is within hydrogen bonding distance.
Salt bridges can contribute to protein stability
They have an electrostatic component in addition to the established
H-bond.
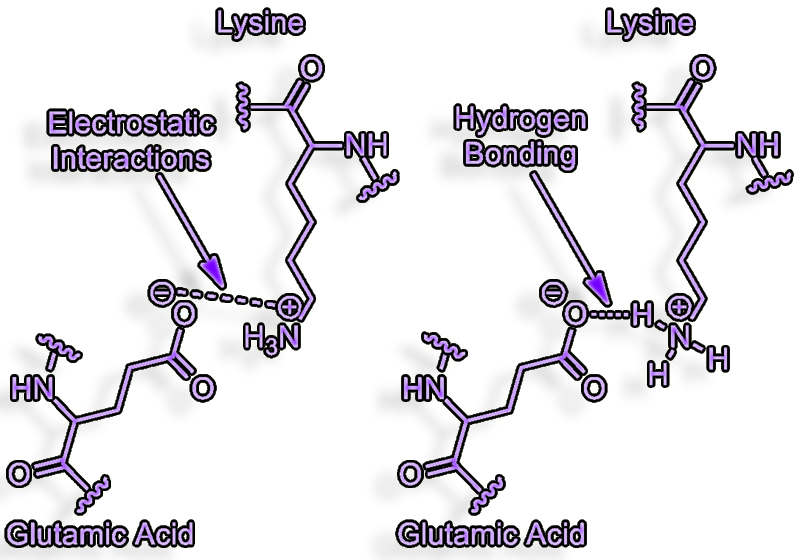
The contributions of salt bridges to free energy
also vary considerably.
They can add up to 4+ kcal/mole to the free energy of binding if they
are generated without solvent shielding, severe entropy penalties, and a
hydrophobic binding site environment (enthalpy)
This is an order of magnitude improvement in binding of about 1000 times
(1.4 kcal/mole).
---- Summary ----
As of now you know all basics of all the laws of thermodynamics.
-
Hydrophobic effect
-
Salt bridges
-
Induced fit
-
etc..
________________________________________________________________________________________________________________________________

________________________________________________________________________________________________________________________________
